Ovaska Research Group

Our research focuses on two main areas: 1) the development of novel methods and strategies for the preparation of complex polycyclic ring systems, and 2) application of these methods for the synthesis of biologically important natural products. We have been particularly interested in natural products that contain seven-membered (cycloheptane) rings. Compounds that incorporate seven-membered rings are widespread in nature and frequently of considerable medicinal interest as potential anti-tumor, anti-HIV, antibacterial and anti-inflammatory agents. More recently we have also investigated strategies for the generation of eight-membered ring-containing systems, which are also prevalent in nature but challenging to access by synthetic means.
Methodology
We have found that a “one-pot” 5-exo cyclization/Claisen rearrangement sequence is a very efficient process that allows the preparation of a number of cycloheptanoid ring systems. The reaction sequence involves an appropriately substituted 4-alkyn-1-ol system which, in the presence of catalytic base and heat, undergoes a unique oxyanionic cyclization reaction to provide an exocyclic tetrahydrofuran derivative. On continued heating, this intermediate rearranges spontaneously via the 3,3-sigmatropic process to afford the final product. Although the early studies were conducted using conventional heating, we found that microwave irradiation (MWI) is particularly well suited for this process.
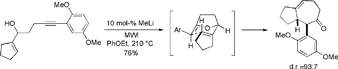
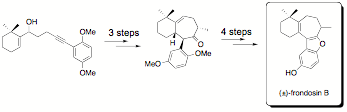
Using a similar strategy, we have recently demonstrated that a variety of cyclooctenone derivatives may be prepared from appropriately substituted 5-alkyn-1-ol systems via microwave-assisted oxy-anionic 6-exo dig cyclization/Claisen rearrangement reaction sequence in the presence of catalytic base. However, the initial 6-exo cyclization is sluggish and requires activation of the triple bond. We found that cyano-substituted 5-alkyn-1-ols are particularly well suited as starting materials for these reactions.
Synthetic Targets
A partial list of natural product targets currently pursued in our laboratory is shown below. So far we have successfully synthesized (±)-frondosin C, (±)-frondosin A (formal total synthesis) and both enantiomers of frondosin B by applying an asymmetric strategy for the synthesis of the requisite 4-alkyn-1-ol precursor.
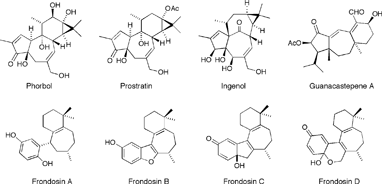
The phorbols are representative of the unique tetracyclic 5-7-6-3 tigliane class of natural products. Although phorbol itself is only moderately bioactive, its C12, C13 diesters are extremely potent activators of protein kinase C (PKC), the phosphorylating enzyme, which has been shown to play a major role in cellular signal transduction. Signal transduction is an important regulatory process that mediates cell growth and division including the types of abnormal cell proliferation associated with tumor promotion. Therefore, compounds capable of interacting with PKC are of great interest as they provide unique opportunities for research on tumor development and cancer.
Interestingly, there are some close structural analogues of phorbol that elicit very different biological activity. An example of these is prostratin, initially isolated from the Samoan medicinal plant Homalanthus Nutans which, unlike phorbol, does not appear to activate PKC; on the contrary, it has been shown to inhibit neoplasia and tumor promotion. Moreover, prostratin is currently being investigated as a possible anti-HIV agent. At noncytotoxic concentrations, prostratin was found to prevent reproduction of the HIV-1 virus in lymphocytic and monocytic target cells. The anti-viral properties of Homalanthus Nutans have been known for a very long time. A brew prepared from the stem wood of this plant containing prostratin as the active ingredient has been used for generations by traditional Samoan healers to treat yellow fever, a viral disease.
Structurally related ingenol derivatives have also been shown to exhibit anti-HIV activity as well as tumor-promoting properties similar to those belonging to the phorbol family. The synthesis and investigation of structurally diverse and modified derivatives of the phorbol family of compounds should ultimately permit full assessment of the features necessary for biological activity, and potentially lead to the development of novel therapeutic agents having anti-cancer and anti-viral activity.
Guanacastepene A is a unique, naturally-occurring diterpene initially isolated from Costa Rican endophytic fungi, possesses significant antibacterial activity toward methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium bacteria. Thus, it serves as a potential lead compound for a new class of antibacterial agents. In addition to guanacastepene A, researchers have identified 14 structurally related compounds that together make up the guanacastepene family of natural products.
Five structurally related sesquiterpene hydroquinones, frondosins A-E, were isolated in 1997 from the Micronesian marine sponge dysidea frondosa . All members of the frondosin family (A-E) are antagonists of interleukin-8 (IL-8) and inhibitors of protein kinase C (PKC) in the low micromolar range. In addition to being involved in cellular inflammatory events, IL-8 is now known to also play an important role in tumor progression and metastasis in several human cancers, including lung cancers. It is has also been reported that IL-8, along with growth-regulated oncogene alpha, is involved in chemoattraction, neovascularization and stimulation of HIV-1 replication both in T-lymphocytes and macrophages. Importantly, it was recently demonstrated that compounds, which inhibit the actions of IL-8 also inhibit HIV-1 replication. Overall, inhibitors of IL-8 action hold therapeutic potential as novel anti-inflammatory and antiviral agents, and may prove useful against cancer as inhibitors of tumorigenesis and proangiogenesis.
Selected Publications
- "A Convenient Route to Fused 5-7-6 Tricyclic Ring Systems", Ovaska, T. V; Roark*, J. L.; Shoemaker*, C. M.; Bordner, J. Tetrahedron Lett . 1998 , 39 , 5705.
- "Synthesis of Octahydro 1H-Pyrrolo[1,2-a]indol-3-ones Via Intramolecular Diels-Alder Reaction of 5-Substituted N-Dienyl Lactams" Smith, M. B.; Ovaska, T.V.; Grant-Young, K.; Wang, C. Synth. Commun. 1998 , 28 , 4233.
- "Tandem Anionic 5-Exo Dig Cyclization-Claisen Rearrangement as an Efficient Route to Fused Polycyclic Ring Systems" Ovaska, T. V.; Roses*, J. B. Organic Letters 2000 , 2 , 2361.
- "Facile Entry to the Tetracyclic 5-7-6-3 Tigliane Ring System " Ovaska, T. V.; Reisman*, S. E.; Flynn*, M. A. Organic Letters 2001 , 3 , 115.
- "Facile approach to the bicyclo[5.3.0]decane ring system; efficient synthesis of (+-)-7-epi- b -bulnesene" Ovaska, T. V.; Ravi Kumar, J. S.; Hulford, C. A.*; O'Sullivan, M. F.*; Reisman, S. E.* Tetrahedron Letters 2002 , 1939.
- "A Mild and Efficient Synthesis of Oxindoles: Progress Towards the Synthesis of Welwitindolinone A Isonitrile" Ready, J. M; Reisman, S. E.; Hirata, M; Weiss, M. M.; Tamaki, K.; Ovaska, T. V.; Wood, J. L. Angew. Chem. Int. Ed. Eng. 2004 , 43 , 1270.
- "Microwave Enhanced Tandem 5-exo Cyclization/Claisen Rearrangement Reactions: a Convenient Route to Cycloheptanoid Ring Systems" McIntosh, C. E.*; Martinez, I; Ovaska, T. V. Synlett 2004 , 2579.
- " First Approach to the Frondosin C Ring System via a Tandem Cyclization/Claisen Rearrangement Sequence" Martinez, I.; Alford, P. E.*; Ovaska, T. V. Org. Lett. 2005 , 7 , 1133.
- "Progress toward the Total Synthesis of Frondosin C" Li, X.; Kyne, R. E.*; Ovaska, T. V. Org. Lett. 2006 , 8 . 5153.
- " Total Syntheses of (±)-Frondosin C and (±)-8- epi -Frondosin C via a Tandem Anionic 5-Exo Dig Cyclization-Claisen Rearrangement Sequence" Li, X.; Kyne, R. E.*; Ovaska, T. V. Tetrahedron 2007 , 63 , 1899.
- "Synthesis of Seven-Membered Carbocyclic Rings via a Microwave-Assisted Tandem Oxyanionic 5- exo dig Cyclization-Claisen Rearrangement Process" Li, X.; Kyne, R. E.*; Ovaska, T. V. J. Org. Chem. 2007 , 72 , 6624.
- "Total Synthesis of (±)-Frondosin B" Li, X.; Ovaska, T. V. Org. Lett. 2007 , 9 , 3837.
- "Intramolecular Thermal Alleneyne 2+2 Cycloadditions; Facile Construction of the 5-6-4 Ring Core of Sterpurene”, Ovaska, T. V.; Kyne, R. E* Tetrahedron Lett. 2008, 49, 376.
- "Evolution of Synthetic Strategy: Total Synthesis of (±)-Welwitindolinone A Isonitrile” Reisman, S. E.; Ready, J. M.; Weiss, M. W.; Hasuoka, A.; Hirata, M.; Tamaki, K.; Ovaska, T. V.; Smith, C. J.; Wood. J. L. J. Am. Chem. Soc. 2008, 130, 2087
- "Studies toward Frondosin A and Its Analogues. Formal Total Synthesis of (±)-Frondosin A” Li, X.; Keon, A. E.*; Sullivan, J. A.*; Ovaska T. V. Org. Lett. 2008, 10, 3287.
- "Asymmetric Synthesis of Seven-Membered Carbocyclic Rings via a Sequential Oxyanionic 5-Exo Dig Cyclization/Claisen Rearrangement Process. Total Synthesis of (–)-Frondosin B” Ovaska, T. V.; Sullivan, J. A.*; Ovaska, S. I.; Winegrad, J. B.*; Fair, J. D. Org. Lett. 2009, 11, 2715.
- "Synthesis of Cycloheptanoid Natural Products via a Tandem 5-Exo-Cyclization/Claisen rearrangement process." Ovaska. T. V. Arkivoc 2011, 34.
- "Facile Access to Cyclooctanoid Ring Systems via Microwave-Assisted Tandem 6-exo dig Cyclization-Rearrangement Sequence," Feldman, A. W.*; Ovaska, S. I.; Ovaska, T. V. Tetrahedron 2014, 70, 4147.
* Student co-authors indicated by asterisk
Funding for Research

National Institutes of Health NIGMS
2000-2003, 2003-2006, 2006-2009, 2009-2013, 2014-2017

ACS Petroleum Research Fund G and AC
2000-2002, 2002-2005

Research Corporation
2000-2002

Camille and Henry Dreyfus Foundation
(Scholar-Fellow Program) 2003-2005
Selected Presentations
- "Tandem Cyclization/Claisen Rearrangement Approach to the Synthesis of Cycloheptanoid Natural Products" invited seminar, presented at the University of Connecticut, Storrs, CT, March 2003
- "Tandem Cyclization/Claisen Rearrangement Approach to the Synthesis of Hydroazulenic Natural Products", invited presentation at the International Komppa Symposium, Helsinki, Finland, July 2003.
- "Tandem 5-exo dig Cyclization/Claisen Rearrangement Strategy as a Route to Polycyclic Cycloheptanoid Natural Products" invited seminar, presented at the University of Turku, Turku, Finland, October, 2004.
- "Tandem Cyclization/Claisen Rearrangement Approach to the Synthesis of Cycloheptanoid Natural Products" invited seminar, presented at Trinity College, February, 2004.
- "Tandem 5-exo dig Cyclization/Claisen Rearrangement Strategy as a Route to Polycyclic Cycloheptanoid Natural Products", invited lecture at Dynea Chemicals/Perstorp AB, Porvoo, Finland, March 2004.
- "Tandem Cyclization/Claisen Rearrangement Strategy as a Route to Polycyclic Cycloheptanoid Natural Products", invited presentation at the Conference of the Latest Trends in Organic Synthesis (LTOS), St. Catherines, Canada, August, 2004.
- "A Novel Approach to the Frondosins via a Tandem Cyclization/Claisen Rearrangement Sequence" presented by Dr. Isamir Martinez at the American Chemical Society National Meeting in San Diego, CA, March 2005.
- "A Novel Approach toward the Synthesis of the Frondosins", keynote presentation at the 14 th European Symposium on Organic Chemistry, Helsinki, Finland, July, 2005.
- "Microwave Assisted Tandem Cyclization/Claisen Rearrangement Strategy as a Route to Cycloheptanoid Natural Products", invited presentation at Wesleyan University, April, 2006.
- "Microwave Assisted Tandem Cyclization/Claisen Rearrangement Strategy as a Route to Cycloheptanoid Natural Products", invited presentation at Bayer Pharmaceuticals, May, 2006.
- "Microwave Assisted Tandem Cyclization/Claisen Rearrangement Strategy as a Route to Cycloheptanoid Natural Products", invited presentation at Bristol-Myers Squibb, October, 2006.
- "Total Syntheses of (±)-Frondosin C and (±)-8-epi-Frondosin", presented at the 233rd National Meeting of the American Chemical Society, Chicago, Il, March 2007.
- "Oxyanionic 5-exo dig Cyclization/Claisen Rearrangement Reactions: Applications to the Synthesis of Cycloheptanoid Natural Products", presented at the 15 th European Symposium on Organic Chemistry, Dublin, Ireland, July, 2007.
- "Oxyanionic 5-exo dig Cyclization/Claisen Rearrangement Reactions: Applications to the Synthesis of Cycloheptanoid Natural Products”, invited seminar at the University of Pennsylvania, February 2008.
- "Total Synthesis of (±)-Frondosin A”, presented at the 235th American Chemical Society National Meeting in New Orleans, LA, April 2008.
- "Oxyanionic 5-exo dig Cyclization/Claisen Rearrangement Reactions: Applications to the Synthesis of Cycloheptanoid Natural Products”, invited seminar at West Virginia University, January 2009.
- "Oxyanionic 5-exo dig Cyclization/Claisen Rearrangement Reactions: Applications to the Synthesis of Cycloheptanoid Natural Products”, invited seminar at Pittsburgh University, January, 2009.
- "Tandem Cyclization/Claisen rearrangement Strategies for the Construction of Polycyclic Natural Products”, invited seminar at the University of Helsinki, Finland, August, 2009.
Selected Student Presentations
- "Asymmetric Synthesis of the Tetracyclic Carbon Framework of Phorbol", Undergraduate Research Symposium, Pfizer Central Research, Groton, CT, October 2000. Presented by Sarah Reisman, '01.
- "Facile Entry to the Fused Tetracyclic 5-7-6-3 Tigliane Ring System", Randolph T. Major Symposium, University of Connecticut, Storrs, CT, October 2000. Presented by Sarah Reisman, '01.
- "Base and Palladium Catalyzed 5-Exo Dig Cyclization/Claisen Rearrangement as a Route to Fused Polycyclic Ring Systems", Boehringer-Ingelheim Fellows Symposium, Boehringer-Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, October 2000. Presented by Michael O'Sullivan, '01.
- "Base and palladium catalyzed 5-exo dig cyclization/claisen rearrangement as a route to fused polycyclic ring systems", presented by Michael O'Sullivan '01, at the 221st National Meeting of the American Chemical Society, San Diego, CA, March 2001.
- "An expedient route to the fused tetracyclic 5-7-6-3 ring system of phorbol", presented by Sarah Reisman '01, at the 221st National Meeting of the American Chemical Society, San Diego, CA, March 2001.
- "Novel Strategies for the Synthesis of the Phorbol Core", presented by Adilah Bahadoor, '02, at the National Meeting of the American Chemical Society, Orlando, FL, March 2002.
- "Novel Synthetic Route to the Hydroazulenic Lactone Natural Products"", presented by Catherine Hulford, '02, at the National Meeting of the American Chemical Society, Orlando, FL, March 2002.
- "Synthesis of the Phorbol Ring System via a Consecutive 5-exo-dig Cyclization/Claisen Rearrangement", presented by Kyle Parcella, '05 at Pfizer Summer Undergraduate Fellowship event, October, 2004.
- "A New Approach to the Frondosins via a Tandem-Cyclization/Claisen Rearrangement Sequence", presented by Matt Tyler, '06 at the Bristol-Myers Squibb undergraduate fellowship symposium, January, 2005
- "Progress on the Synthesis of Phorbol Esters via a Consecutive 5-exo-dig Cyclization/Claisen Rearrangement", presented by Kyle Parcella, '05 at the American Chemical Society National Meeting in San Diego, CA, March 2005
- "Microwave-Enhanced Tandem 5-exo-dig Cyclization/Claisen Rearrangement Reactions as a Convenient Route to the Synthesis of the Phorbol Core", presented by Cait McIntosh, '05 at the American Chemical Society National Meeting in San Diego, CA, March 2005.
- "Studies Toward the Total Synthesis of Frondosin C", presented by Robert Kyne, '07 at the Bristol-Myers Squibb undergraduate fellowship symposium, January, 2006.
- "Novel Approach to the Tricyclic Guanacastepene Ring System", presented by George Arab, '07 at the American Chemical Society National Meeting in Atlanta, GA, March 2006.
- "Studies toward Guanacastepene A", presented by George Arab, '07 at the Bristol-Myers Squibb undergraduate fellowship symposium, January, 2007.
- "Novel Approach to Guanacastepene", presented by George Arab, '07 at the American Chemical Society National Meeting in Chicago, Il, March 2007.
- "Strategies toward the Synthesis of the Frondosins" , presented by Robert Kyne, '07 at the American Chemical Society National Meeting in Chicago, IL, March 2007.
- "Studies Toward the Total Syntheses of Frondosins A, B, D and E”, presented by Alec Keon, ’08 at the Bristol-Myers Squibb undergraduate fellowship symposium, January, 2008.
- "Synthetic studies toward the frondosins; total synthesis of (±)-frondosin A”, presented by Alec Keon, ’08 at the 235th American Chemical Society National Meeting in New Orleans, LA, April 2008.
- "Synthesis of optically active seven-membered carbocyclic rings via a microwave-assisted oxyanionic 5-exo dig cyclization/Claisen rearrangement sequence”, presented by Jonathan Sullivan, ’08 at the 235th American Chemical Society National Meeting in New Orleans, LA, April 2008.
Chemistry Department
Mailing Address
Connecticut College
Chemistry Department
270 Mohegan Avenue
New London, CT 06320
Campus Location
Hale 101